Schematic energy levels of a hypothetical atom with configurations d 2... | Download Scientific Diagram

The energy levels of an atom are shown below. Which of them will result in the transition of a photon of wavelength 275 nm?

Suppose that a hypothetical atom gives a red, green, blue and violet line spectrum. Which jump - YouTube

In a hypothetical atom, the number of electrons in shell and subshell are x and y , respectively, where each electron has three possible spin states represented as s = + 1/2, -

OneClass: Be sure to answer all parts. Consider the following energy levels of a hypothetical atom: E...

In a hypothetical atom, the number of electrons in shell and subshell are x and y , respectively, where each electron has three possible spin states represented as s = + 1/2, -
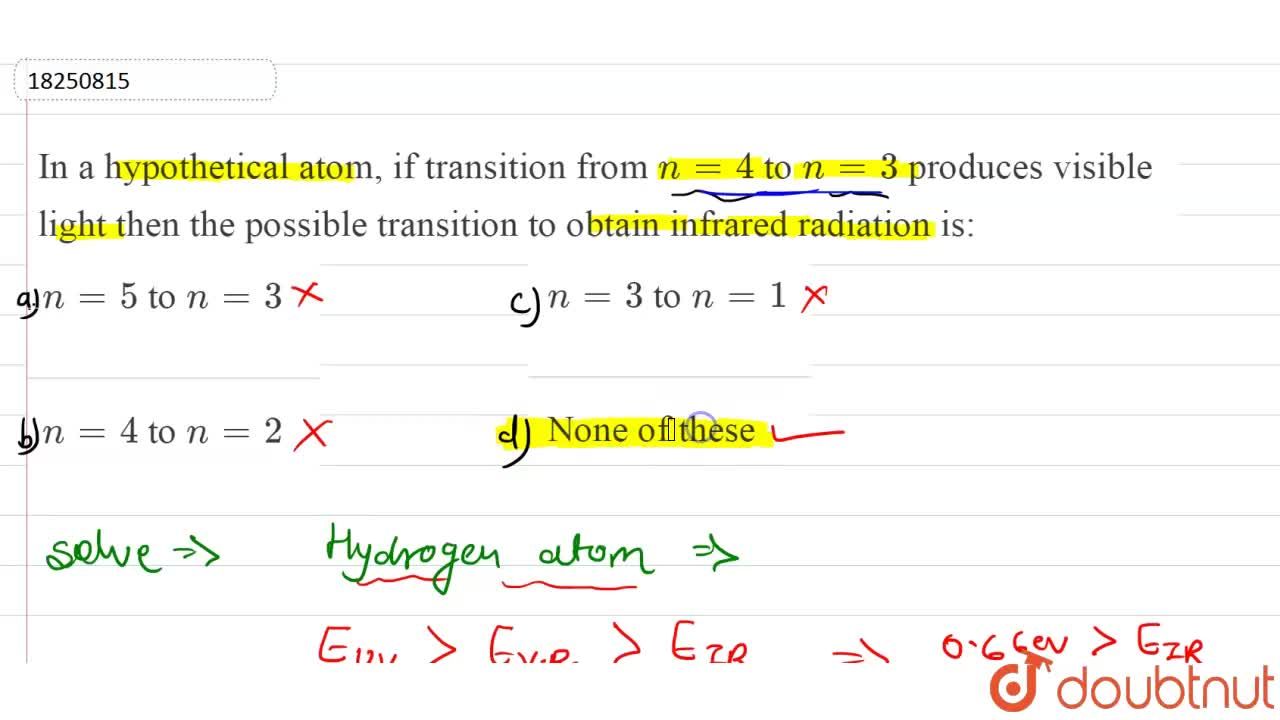
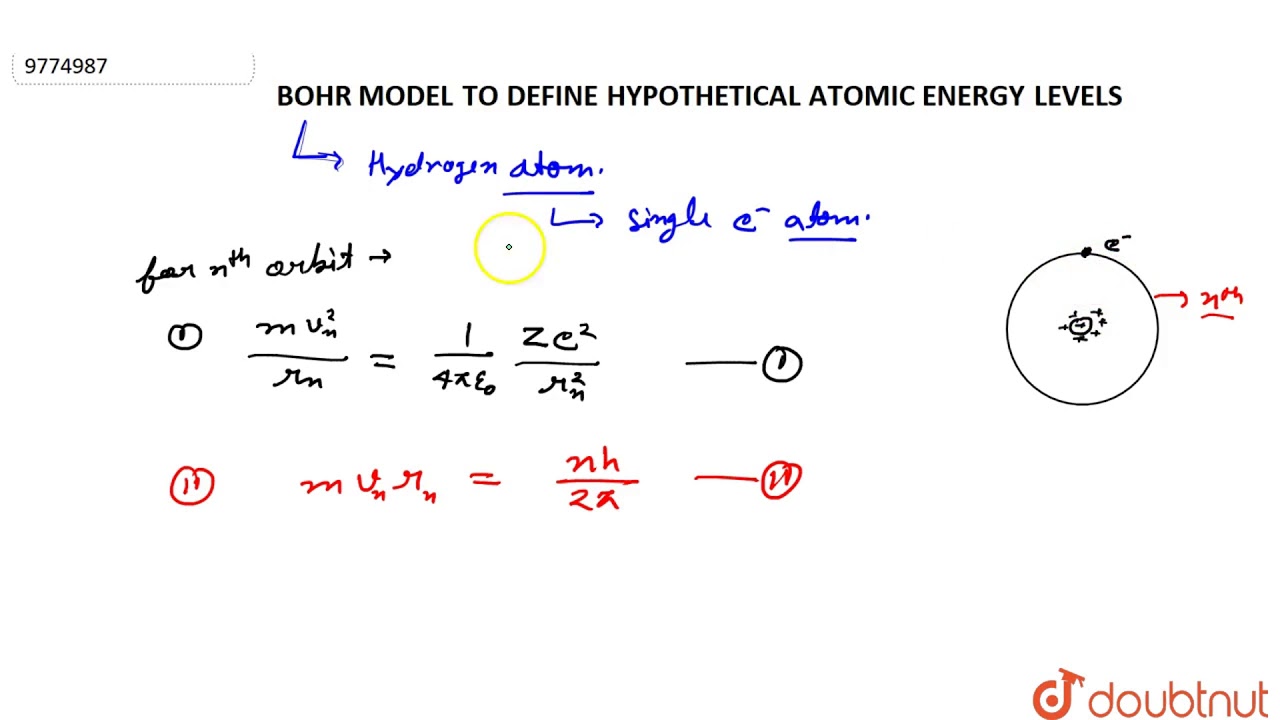
In a hypothetical atom, if transition from n=4 to n=3 produces visible light then the possible transition to obtain infrared radiation is:
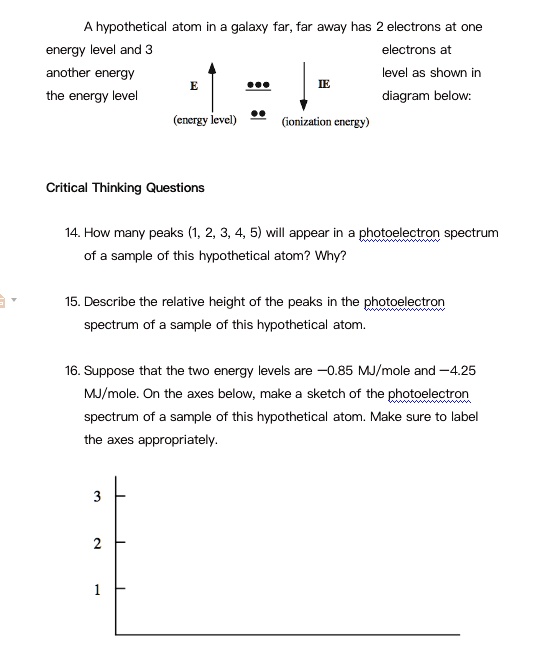
SOLVED: hypothetical atom in galaxy far, far away has electrons at One energy level and 3 electrons at anotner energy the energy level level as shown in diagram below: (ercizy levcl) (ionization

The energy levels of an atom are shown below. Which of them will result in the transition of a photon of wavelength 275 nm?

Suppose that a hypothetical atom gives a red, green, blue and violet line spectrum. Which jump according to figure would give off the red spectral lin - Sarthaks eConnect | Largest Online

SOLVED: A hypothetical atom has only two excited states, at 4.0 and 7.0 eV, and has a ground-state ionization energy of 9.0 eV. If we used a vapor of such atoms for
The energy level diagram is for a hypothetical atom. A gas of these atoms initially in the ground - Sarthaks eConnect | Largest Online Education Community

4: Two-level energy diagram of a hypothetical atom in the presence of a... | Download Scientific Diagram

The energy level diagram is for a hypothetical atom A gas of these atoms initially in the ground state is irradiated with photons having a continuous range of energies between 7and 10

Schematic energy levels of a hypothetical atom with configurations d 2... | Download Scientific Diagram
In a hypothetical atom, the number of electrons inshell and subshell are x and y, respectively, whereeach electron has three possible spin states repre sented as s +1/2, 1/2 and 0.Then the

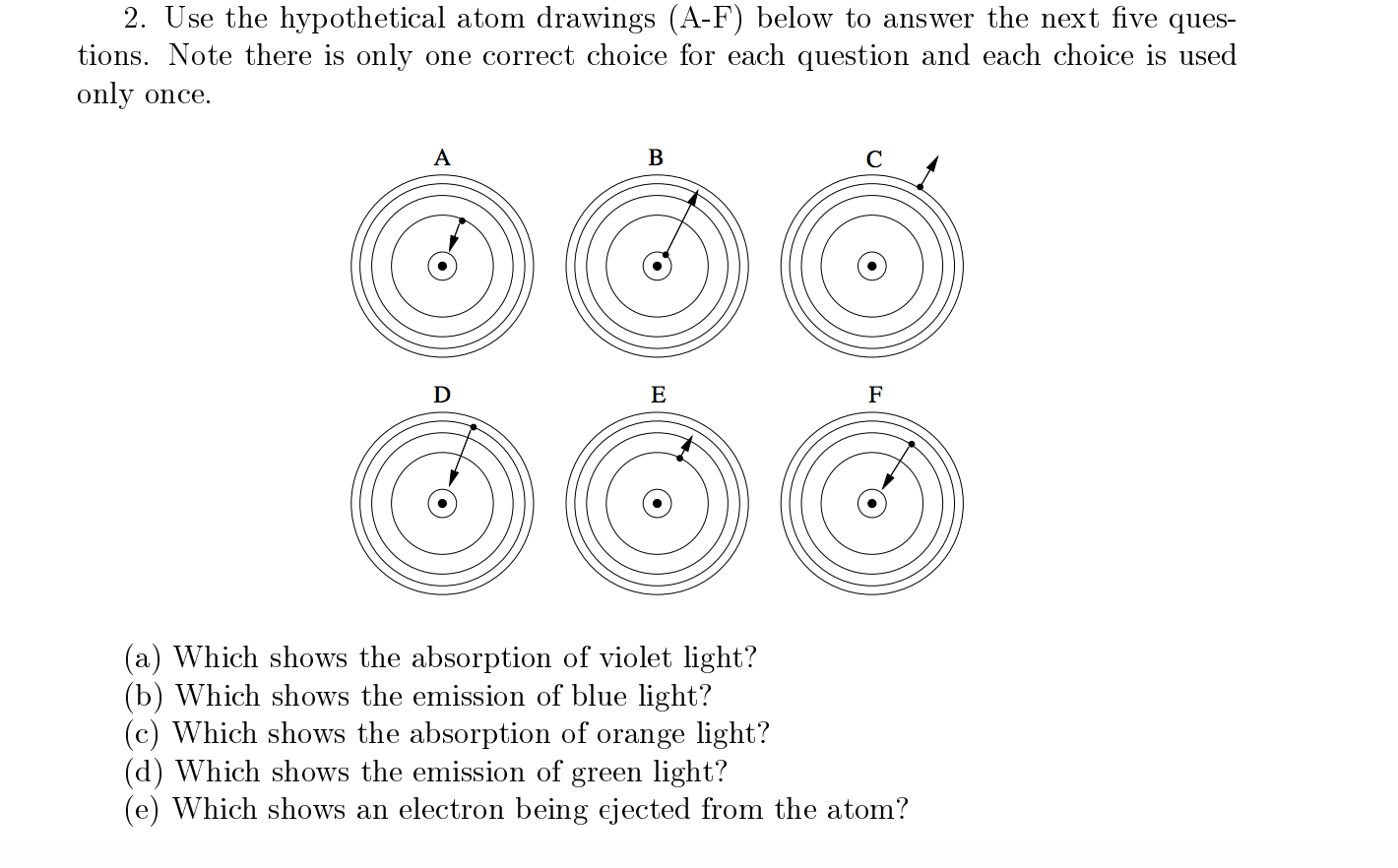
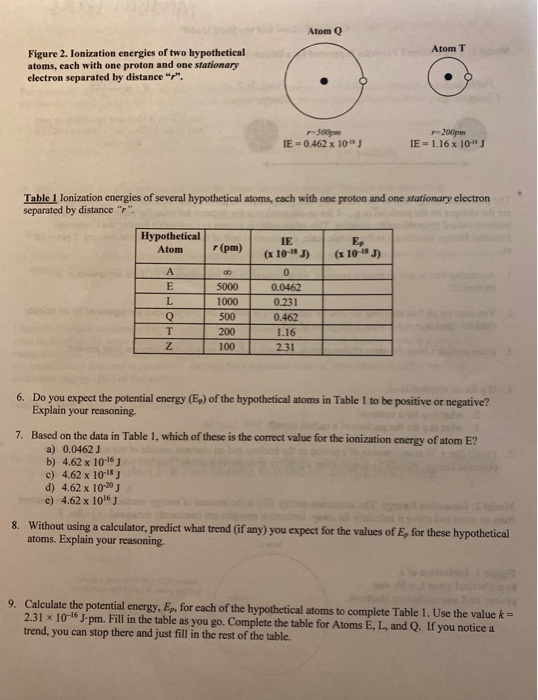
![ANSWERED] Consider the hypothetical atom "X". If the... - Organic Chemistry ANSWERED] Consider the hypothetical atom "X". If the... - Organic Chemistry](https://media.kunduz.com/media/sug-question/raw/45022300-1658615686.1122277.jpeg)